Abstract
Philadelphia (Ph)-like ALL is associated with poor response to standard chemotherapy and frequently necessitates novel salvage therapies to attain remission. However, outcomes of novel therapies in relapsed/refractory (r/r) Ph-like ALL remains largely unknown, except for a few reported small case series. Here, we retrospectively studied outcomes of adult patients (pts) with r/r B-cell ALL who completed at least one cycle of a novel salvage therapy [blinatumomab (blina), inotuzumab (INO), or CD19CAR T cells (CAR)] at City of Hope from 2012 to 2022 and had post treatment disease assessment. We only included pts with documented fusions associated with Ph-like ALL, identified by accumulative results of RNAseq, conventional cytogenetics, FISH, and whole genome SNP array analysis. Complete remission (CR)/CR with incomplete hematologic recovery (CRi) rate was the primary endpoint and leukemia-free survival (LFS) and overall survival (OS) were secondary endpoints. LFS was defined as the period from the date of response to individual therapy to the date of relapse or death, whichever came first; LFS was censored among responders. at the last follow-up if pts were alive and relapse-free.
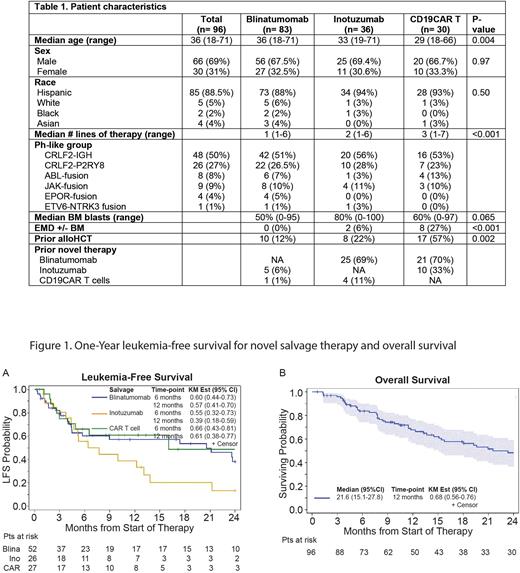
We identified 96 pts with r/r B-cell ALL with Ph-like fusions who received 149 individual novel therapies; 83 (86%) pts were treated with blina, 36 (38%) with INO, and 30 (31%) with CAR [commercial; n=10; investigational: n=20]. Median age at the time of first novel salvage therapy was 36 years (range; 18-71), 69% of pts were males and 89% were Hispanic. Ph-like fusions including IGH-CRLF2, P2RY8-CRLF2, ABL1/2,JAK2-, EPOR- and ETV6-NTRK2-fusion were seen in 50%, 27%, 8%, 9%, 4% and 1% of the cohort, respectively. Pts with CAR therapy had more prior lines of therapy compared to blina and INO (3 vs 1 vs 2; p<0.001), more frequently underwent prior allogeneic transplant (57% vs 12% vs 22%; p=0.002), and had a trend toward more extramedullary disease at the time of initiating therapy (27% vs 0 vs 6%; p= 0.065). Blina was administered at older median age compared to INO and CAR (36 vs 33 vs 29; p=0.004). Pts treated with INO had a trend toward higher marrow blasts% at the time of starting therapy (80 vs 60 vs 50; p= 0.065) compared to CAR and blina, respectively. Prior novel therapies were given for relapse to 7% (n=6), 75% (n=27), and 70% (n= 21) before blina, INO and CAR, respectively.
The CR/CRi rates were 63%, 72%, and 90% after blina, INO and CAR, with corresponding negative minimal residual disease (MRD-) rate among evaluable responders (defined as <0.01% leukemic cells in the bone marrow by multicolor flow cytometry) of 86%, 73%, and 96%, respectively. Among responders, 50%, 50%, and 44% of pts receiving blina, INO or CAR, respectively; underwent consolidation with allogeneic transplant. CR/CRi rates for pts treated with INO with/without prior lines of novel therapy were 74% and 67%, respectively. CR/CRi were 90% and 89% for CAR pts treated with/without prior novel therapies.
The median follow up since the first salvage novel therapy was 13.1 months (range; 0.6-109.5), and the median OS was 21.6 months (range; 15.1-27.8). In multivariable analysis, type of novel therapy (p= 0.044) and marrow blasts [per 5% increase] (p=0.006) independently predicted CR/CRi rate, while the Ph-like fusion subgroup was associated with LFS (p= 0.013), with 6-months LFS of 45%, 67%, and 90% for pts carrying IGH-CRLF2, P2RY8-CRLF2 and other fusions, respectively. Yet, LFS was not different among pts receiving individual novel salvage therapies (p=0.30), and the 6-month LFS for blina, INO and CAR were 60%, 55% and 66%, respectively.
To our knowledge, our study is the largest experience evaluating outcomes of novel salvage therapies for Ph-like ALL. Our results show that novel therapies are highly effective in r/r ALL with Ph-like fusions, leading to high remission rate, MRD- response and transition to transplant consolidation at remission. Prior novel therapies did not adversely impact response to subsequent novel therapies. Therefore, the early introduction of novel therapies in pts with Ph-like ALL is of great interest and could potentially lead to improvement in frontline therapy outcomes in this high-risk entity.
Disclosures
Aldoss:AbbVie: Consultancy, Research Funding; Amgen: Consultancy; Agios: Consultancy, Honoraria; Autolus Limited: Consultancy; Kite: Consultancy; Jazz Pharmaceuticals: Honoraria, Speakers Bureau. Koller:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; Treadwell Therapeutics: Other: Safety Review Committee. Al Malki:CareDx: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Miltenyi Biotec: Consultancy, Research Funding; Hasna Biopharma: Membership on an entity's Board of Directors or advisory committees; NexImmune: Consultancy, Research Funding; Gilead: Consultancy, Research Funding. Salhotra:Kadmon: Other: Advisory board meeting ; BMS: Research Funding; Orca Bio: Research Funding. Aribi:SeaGen: Consultancy. Ball:Oncovalent: Membership on an entity's Board of Directors or advisory committees. Artz:Abbvie: Honoraria; Magenta: Honoraria. Becker:Pfizer Pharmaceuticals: Research Funding; Accordant Health Services (CVS Caremark): Consultancy; Glycomimetics: Research Funding; Notable labs: Research Funding. Smith:Johnson and Johnson: Current equity holder in publicly-traded company. Marcucci:Agios: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings. Stein:Amgen: Speakers Bureau. Nakamura:Kadmon: Consultancy; Omeros: Consultancy; Helocyte Inc: Research Funding; Magenta Therapeutics: Consultancy; Sanofi: Consultancy; BluebirdBio: Consultancy. Pullarkat:Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member; AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.